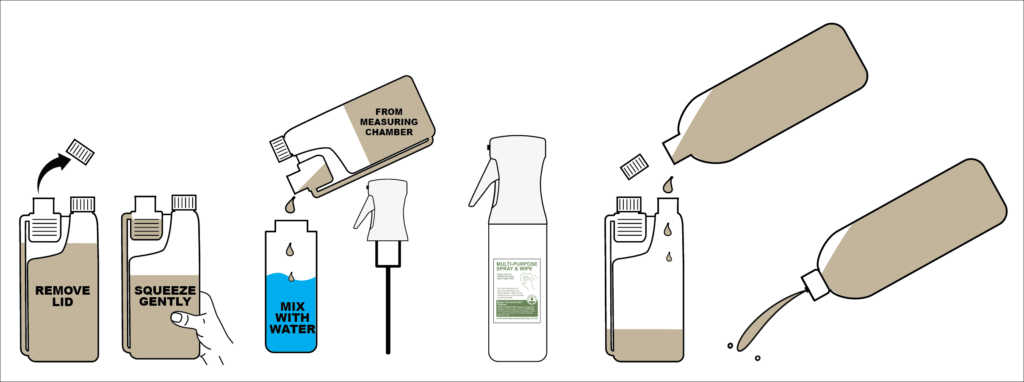
How to Use
How to dilute and use the measuring chamber.
- Make sure the cap for the main chamber is on and airtight.
- Remove the cap from the small chamber.
- Squeeze the main bottle until the required amount of liquid is in the small chamber.
- Pour from the small chamber into your bottle or bucket.
- Slowly top the bucket or bottle with water to avoid foam overfilling the bottle.
- Shake or stir well, and it is ready to use.
How to use the 1 litre bottle
- When your 500mL measuring bottle runs out use the 1 litre bottle refill to fill the large chamber.
- Use the squirt flip cap to apply the concentrated solution directly onto heavily soiled areas.
- Scrub and wash off with clean water.
DILUTION RATE
|
APPLICATION
|
DILUTION mL / Litre
|
|
Disinfecting
|
Use Undiluted
|
|
Sanitising Spray
|
125mL with 375mL water
|
|
Toilets & Odour Control
|
50mL with 450mL water
|
|
Degreasing
|
50mL with 450mL water
|
|
Pet Messes & Odour Control
|
50mL with 450mL water
|
|
Floors
|
125mL with 5 Litres water
|
|
General Spray & Wipe
|
25mL with 475mL water
|
DIRECTIONS HOW TO USE
Disinfectant: First, remove heavy dirt from the surface. Then, apply the disinfectant undiluted with a spray or a cloth, ensuring the entire surface is covered. Leave for 10 minutes, then wipe off with a damp cloth. Rinse food-contacting surfaces with clean water before use.
Sanitising: Apply with spray or a cloth. Ensure the entire surface is covered and allow it to dry.
Toilets, Urinals & Odour Control: Spray the area, soak for 2-3 minutes, scrub, and wash down. For heavily soiled areas, soak with the undiluted solution for up to 30 minutes before scrubbing.
Pet Messes & Odour Control: Spray the area, soak for 2-3 minutes, scrub, and wash down. For heavily soiled areas, soak with the undiluted solution for up to 30 minutes before scrubbing.
Degreasing: Spray onto the surface, wait 30 seconds, scrub if required, then wipe with a clean cloth.
Floors: Mop the floor and allow it to dry. After mopping, pour the balance down the drain. This will help keep your drain clean.
Multi-purpose Spray & Wipe: Spray onto the surface and wipe with a clean cloth.
Note: Heavily soiled areas may need repeat treatments. Universal 6in1 Cleaner in concentrate form has a pH of 3.6 (acidic) and could etch some stone surfaces. It is not recommended to use the concentrate on stone benchtops. Always test in an inconspicuous area before use.
Safety & First Aid
FRAGRANCE: Tassie Mint
ACTIVE INGREDIENTS: 0.06%w/w Thymol.
Non- Flammable
SAFETY INFORMATION
Keep out of reach of children. Avoid contact with eyes.
Rinse food-contacting surfaces with clean water before use.
FIRST AID
If swallowed contact a Doctor or Poisons Information Centre (e.g. phone Australia 13 11 26; New Zealand 0800 764 766). If medical advice is needed, have product container or label at hand.
The Science Behind the Product
- Surfactin: https://www.sciencedirect.com/science/article/pii/S0927776521001934
Highlights
- High-purity surfactin was recovered from a Bacillus subtilis culture broth.
- Purified surfactin efficiently reduces interfacial tension at low concentrations.
- Surfactin was characterized regarding its applicability as O/W emulsifier.
- Emulsions were stable at pH values above 6 and NaCl concentrations up to 0.5 M.
- Emulsions had highly negative zeta-potentials up to −100 mV.
- Thymol: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5483461/
Thymol, chemically known as 2-isopropyl-5-methylphenol is a colourless crystalline monoterpene phenol. It is one of the most important dietary constituents in thyme species. For centuries, it has been used in traditional medicine and has been shown to possess various pharmacological properties including antioxidant, free radical scavenging, anti-inflammatory, analgesic, antispasmodic, antibacterial, antifungal, antiseptic and antitumor activities.
- Biosurfactants: https://www.sciencedirect.com/topics/medicine-and-dentistry/biosurfactant
Biosurfactants are active compounds that are produced at the microbial cell surface or excreted and reduce surface and interfacial tension. Microbial surfactants offer several advantages over synthetic ones, such as low toxicity and high biodegradability, and remain active at extreme pH and salinity. Biosurfactants are produced by bacteria, yeasts, and filamentous fungi.
- Understanding Biosurfactants and their use: Video https://www.youtube.com/watch?app=desktop&v=6fm8f3k1FZ0
- The Chemistry of Cleaning – How Surfactants Work: https://www.cleaninginstitute.org/understanding-products/science-soap/chemistry-cleaning
In order to clean dirt off our clothes and surfaces, the water needs to be able to reach the surface. Water is able to get to the surface if surface tension is reduced. To do this, we use a group of chemicals called surface active agents, or surfactants. Surfactants change how water behaves. When a surfactant is added, the surface tension is reduced. Now water can spread out and wet the surface (e.g., clothes, dishes, counter tops) we are trying to clean.
Every surfactant has two ends. One end wants to be in water and the other does not. How these two ends interact with soil and water is the secret to how a surfactant works. Once the surfactant is added to water, the water-fearing ends try to stay away from the water.3,4 Watch this video4 to see how… https://youtu.be/F7-ie4uWX04
- How Surface Tension is Measured: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8433748/
The critical micellar concentration (cmc) is a fundamental property of a surfactant solution. At surfactant concentrations near and above the cmc the surfactant molecules start to aggregate and form micelles. The formation of micelles affects many properties of a surfactant solution introducing usually a marked change at the cmc in plots of a property versus the surfactant concentration.
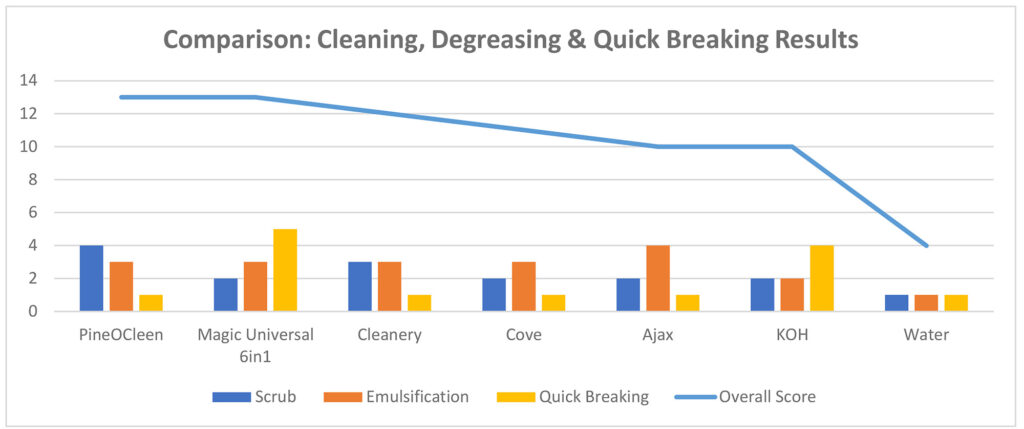
- What is Quick Breaking, and why is it important
https://cleanawater.com.au/information-centre/how-quick-break-degreasers-work
Many water authorities require oil water separators as a pre-treatment for wastewater before it is discharged into the sewer system. This is aimed at protecting downstream water processing plants from excessive oil and grease, and preventing hydrocarbon spills into the environment.
However, oil water separators only work when the wastewater is free from emulsions. This is where quick break degreasers are effective.
How quick-break degreasers work
Degreasers dissolve hydrocarbons in water in the form of an emulsion. They break oil down into tiny droplets that mix in between the water droplets.
- An emulsion keeps oil droplets so small that they do not separate from the water.
- The result is a dirty, foamy-looking wastewater.
- It is impossible to tell how much oil is trapped in the emulsion.
Quick-break degreasers perform this function, then quickly release the oil droplets from the emulsion.
- Once the emulsion is broken, oil droplets stick together to form larger droplets.
- The larger oil droplets are easier to separate from the water.